
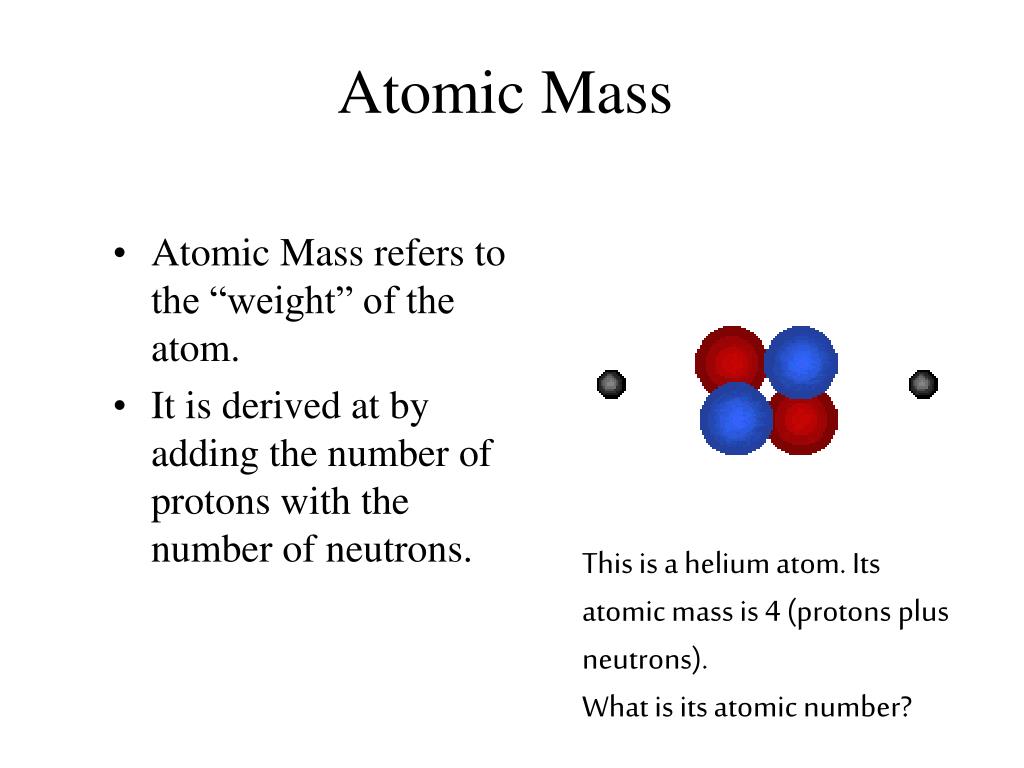
The number of neutrons can be found simply by identifying the atomic mass (found at the bottom of the element on the periodic table) and the atomic number. Its atomic number correlates with the number of protons and electrons the element has, while the element’s neutrons can be determined from its mass. The gaseous element also has an atomic number of 2 and an atomic symbol of He. Helium has an atomic mass of 4.00 atomic mass units. The atomic number is specifically tied to a single element. This number clearly shows you how many protons an element’s nucleus contains. Helium can be found on the periodic table at atomic number two. Superfluid research stable isotopes of helium are helium-3 (or 3He), with two protons and one neutron, and helium-4 (or 4He), with two protons and two neutrons. He3 has two protons, one neutron, and two electrons. Helium can also have two unstable isotopes, helium-6 (four neutrons) and helium-8 (six neutrons). The most common, stable form of helium has two protons and two neutrons. The only exception is helium, which has just two electrons.
#HELIUM PROTONS NEUTRONS ELECTRONS FULL#
Their outer energy levels are full because they each have eight valence electrons. Noble gases are the least reactive of all known elements. Protons and one neutron, and helium-4 (or 4He), with two protons and two neutrons. Helium-4 is made of 2 protons (the part of an atomic nucleus with a positive charge), 2 neutrons (the part of an atomic nucleus with no charge) and 2 electrons (the part of an atom that goes around the nucleus with a negative charge). How many protons neutrons and electrons does helium 4 have? 7 How do you calculate the number of protons and neutrons?.4 How many electrons does helium-3 have?.3 How many neutrons does helium 8 have?.1 How many protons neutrons and electrons does helium 4 have?.So one day, Earth may run out of helium, or at least have to spend more money to find new places where there’s still some helium left. Radioactive decay makes more helium, but not as fast as we’re using it up. There’s a limited amount of helium on earth, and when we use it, it floats away into the atmosphere. Welders, for example, use a lot of helium this way. So if you are working with materials that might explode, you can do it more safely in a helium atmosphere.

Helium atoms are very stable – it’s hard to get them to combine with other atoms into molecules. Helium and explosionsīut the main thing people use helium for is to keep things from exploding. Like hydrogen, helium is lighter than air, so when you fill balloons with helium, they float.
What is uranium? More about electrons And radioactivityīecause helium atoms are small, they are very light. Still underground, the alpha particles find electrons and join up with them to become helium atoms. The helium gets underground when radioactive atoms like uranium (that are underground) decay and shoot off alpha particles, which are the same as helium atoms without their electrons. What are stars made of? Turning into carbon Where on earth do we find helium?īut the helium people use on Earth mainly comes from mining underground gas pockets of helium. When a star runs out of hydrogen, it begins to turn helium atoms into carbon atoms instead. So stars are made mainly of hydrogen and helium atoms. The star makes helium by squashing four hydrogen atoms together into one new helium atom. More about hydrogen What are atoms made of? All our chemistry articles Stars and helium
The only atom simpler than helium is hydrogen. Around the nucleus, there are two electrons. The nucleus of a helium atom has two protons and two neutrons.
Diagram of a helium atom Helium is a very simple atom.


 0 kommentar(er)
0 kommentar(er)
